The term “bond” is used to refer to the sharing of electrons between two atoms. This can involve the transfer of electrons from one atom to another or the sharing of electrons between atoms. A bond is typically formed when two atoms are attracted to each other due to their opposite charges. The strength of the bond is determined by the number of electrons involved in the sharing.
Hybridization is the process by which atoms of different elements combine to form molecules. This is usually done by combining two or more atoms of different elements in a specific way to create a stable structure. Hybridization is necessary in order to create molecules that have properties different from those of the component atoms.
Resonance is the phenomenon in which a molecule can have multiple valid structures. This occurs when the electrons are shared between multiple atoms in different ways. Resonance is important because it allows molecules to have different properties than what would be expected from the component atoms alone. In summary, a bond is the sharing of electrons between two atoms, hybridization is the combination of different atoms to form molecules, and resonance is the phenomenon in which a molecule can have multiple valid structures. These concepts are essential for understanding how molecules interact and behave in different environments.
Bonds, hybridization and resonance are three distinct concepts in chemistry that are related to the structure and behavior of molecules. Each term has a distinct definition and understanding them is essential to understanding the molecular structure of various compounds.
Bonds are the forces that hold atoms together in molecules. These forces can be either covalent, where electrons are shared between the atoms, or ionic, where one atom donates electrons to another. These bonds are responsible for the overall shape and stability of molecules.
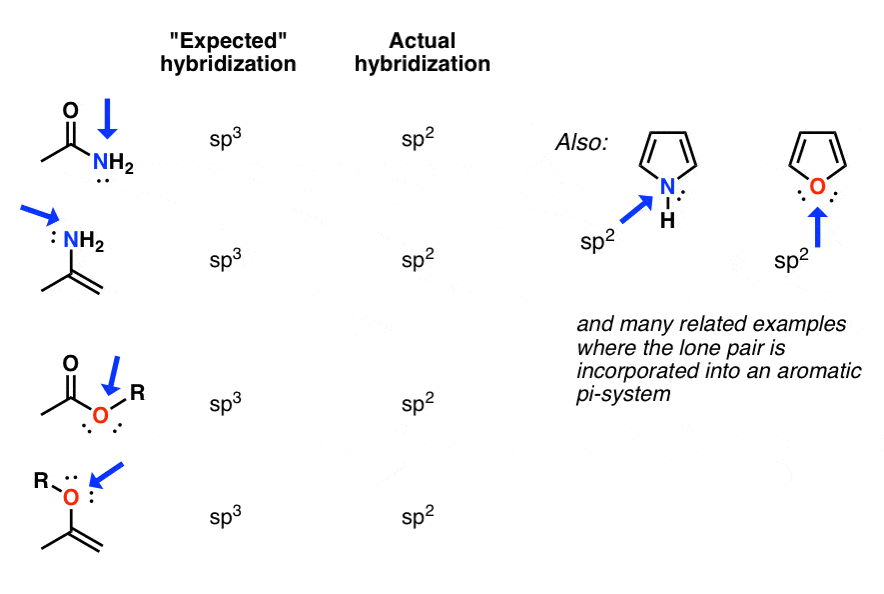
Hybridization is the process of mixing different atomic orbitals to form new orbitals. This is important in understanding how atoms interact with each other. For example, sp3 hybridization is used to explain how two carbon atoms will interact with each other, forming a double bond.
Resonance is a phenomenon where multiple Lewis structures can be used to explain the same molecule. These structures are all valid, but none of them is a perfect representation of the molecule. This phenomenon is found in many molecules, most notably in aromatic compounds.
In conclusion, bonds, hybridization and resonance are three distinct concepts in chemistry that are related to the structure and behavior of molecules. Understanding them is essential for accurately predicting the structure and behavior of compounds.
10